Products
News
Kogene provides a powerful solution
for a molecular diagnostics

SARS-CoV-2
PowerChek™ 2019-nCoV Real-time PCR Kit
The 1st EUA by Korea CDC, MFDS | US FDA EUA approved | CE-IVD
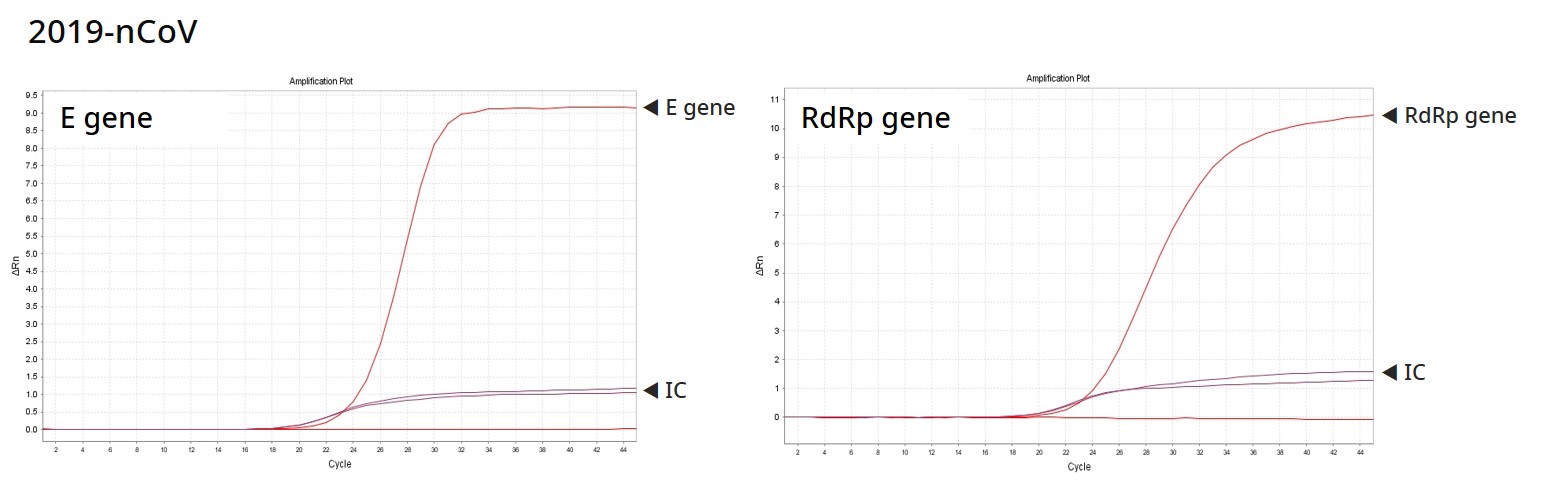
PowerChek™ 2019-nCoV Real-time PCR Kit provides the fast and accurate testing solution for COVID-19, targeting the E gene for Sarbecovirus and the RdRp for COVID-19 specifically. KogeneBiotech is engaged with relevant organizations in Korea, to supply the test kits and offer additional respiratory viral detection solutions for diagnosis by exclusion.
Analytes : Sarvecovirus (E gene), SARS-CoV-2 (RdRp gene), IC
Features
- Accurate confirmation & pool tests
- Highly sensitivity & specificity
- Including all necessary reagents
- Easy to use
Components
- Primer/Probe Mix
- RT-PCR Premix
- Control
- Kit Manual
Result
Product List
| Cat No. | Product | Size | Price | Add to list |
|---|---|---|---|---|
| R6900TD | PowerChek™ 2019-nCoV Real-time PCR Kit | 100 | Request a quote |
Related Product
| Cat No. | Product | Size | Price | Add to list |
|---|---|---|---|---|
| KQ0002 | PowerAmp96™ Dx | 96 wells | Request a quote | |
| E0007 | PowerPrep™ Viral DNA/RNA Extraction Kit | 50 preps | Request a quote | |
| CL0001 | PowerCleanser™ DNA Remover | 500 mL | Request a quote |
Note
PowerChek™ 2019-nCoV Real-time PCR Kit has not been FDA cleared or approved. This test has been authorized by FDA under an emergency use authorization for use by authorized laboratories. This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the authorization is terminated or revoked sooner.
